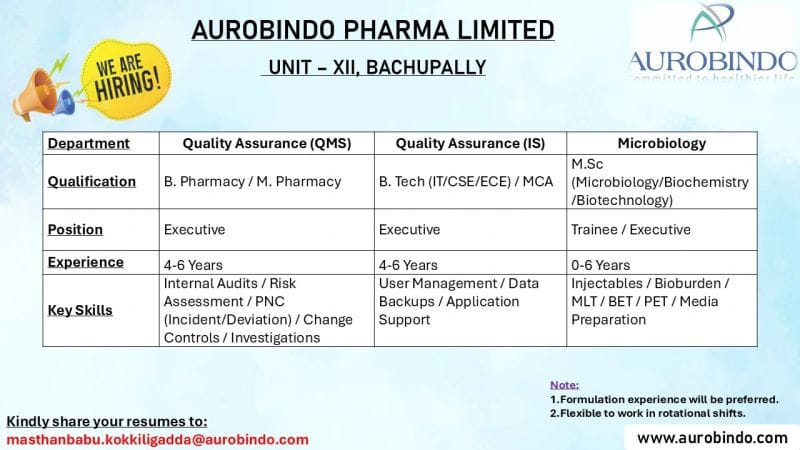
Aurobindo Pharma Limited, a global pharmaceutical company with a focus on providing high-quality medicines across multiple therapeutic areas, is looking to expand its team. The company has an immediate requirement for candidates in various positions at its Unit-XII in Bachupally, Telangana. If you are passionate about contributing to the pharmaceutical industry, this is an excellent opportunity to join a leading name in the sector.
Company Introduction:
Aurobindo Pharma is one of the largest pharmaceutical manufacturers in India, producing over 300 products across a broad spectrum of therapeutic areas. The company has a presence in over 150 countries and boasts an extensive portfolio of formulations, active pharmaceutical ingredients (APIs), and other healthcare solutions. Aurobindo Pharma is recognized globally for its commitment to quality and innovation, playing a key role in the healthcare industry.
Responsibilities in Job:
- Quality Assurance (QMS & IS):
- Conduct internal audits, risk assessments, and manage PNC (incident/deviation) processes.
- Monitor and manage change controls and investigations to ensure compliance with regulatory standards.
- Ensure adherence to quality management systems in the pharmaceutical manufacturing process.
- Microbiology:
- Perform microbiological tests like bioburden, MLT, BET, PET, and media preparation.
- Ensure that all processes are compliant with industry regulations and standards.
- Troubleshoot any issues related to microbiology, supporting the overall quality control efforts of the organization.
- Technical Support (IT):
- User management and data backup operations for the applications used in the pharmaceutical production process.
- Provide technical support for system applications ensuring seamless operation within the quality assurance framework.
Qualification:
- Quality Assurance (QMS & IS):
- B. Pharmacy / M. Pharmacy or B.Tech (IT/CSE/ECE) / MCA with relevant experience.
- Microbiology:
- M.Sc in Microbiology, Biochemistry, or Biotechnology.
- Candidates should have 4-6 years of relevant experience, particularly in pharmaceutical manufacturing or related industries. Freshers with a relevant background in Microbiology are also welcome to apply for trainee positions.
Key Skills:
- Quality Assurance (QMS & IS):
- Expertise in Internal Audits, Risk Assessment, and Incident/Deviation Management.
- Strong understanding of Change Controls and Investigations in the pharmaceutical industry.
- Microbiology:
- Knowledge in Injectables, Bioburden testing, and other microbiological techniques.
- Proficiency in MLT/BET/PET and media preparation.
- Technical (IT Support):
- Proficiency in User Management, Data Backups, and Application Support.
Note: Formulation experience is preferred for candidates applying for Quality Assurance roles. Flexibility to work in rotational shifts is required for all positions.
How to Apply:
Interested candidates can send their resumes to masthanbabu.kokkiligadda@aurobindo.com. Ensure your resume clearly highlights your qualifications and relevant experience as per the roles mentioned above.